Introduction: New immunotherapies directed against CD38, SLAMF7 or BCMA have significantly improved the outcome of multiple myeloma (MM) patients. However, most patients eventually relapse, underscoring the need for additional immunotherapeutic targets.
We have previously shown that expression levels of GPRC5D, an orphan G protein-coupled receptor, are significantly higher on MM cells, compared to normal plasma cells or other immune cells. We also showed that the novel GPRC5DxCD3 bispecific antibody (BsAb) JNJ-7564, has promising anti-MM activity in patient-derived BM samples (Verkleij et al., EHA 2019). To elucidate which factors contribute to the observed heterogeneity in ex vivo response, we analyzed the impact of tumor and patient characteristics on efficacy of JNJ-7564. We further investigated whether tumor-intrinsic factors may be determinants of response by also testing in these assays JNJ-7957, a BCMA-targeting BsAb that differs from JNJ-7564 only in the tumor-antigen-binding domain.
Methods: Bone marrow (BM) samples obtained from 13 newly diagnosed (ND), 17 daratumumab-naive relapsed/refractory (DARA-naive RR; median of 3 prior therapies) and 15 daratumumab-refractory (DARA-R, median of 6 prior therapies) MM patients were analyzed for tumor- and immune cell composition, and subsequently incubated with JNJ-7564 (0.00128-4.0 µg/mL) or JNJ-7957 (0.8 µg/mL). After 48 hours, MM cell lysis was assessed by flow cytometry.
Luciferase-transduced MM cell lines were incubated with JNJ-7564 (0.032-4.0 µg/mL) in the presence of healthy peripheral blood mononuclear cells (PBMCs), purified CD4+CD25- T-cells or regulatory T-cells (Tregs). After 48 hours, MM cell lysis was assessed by bioluminescence assay.
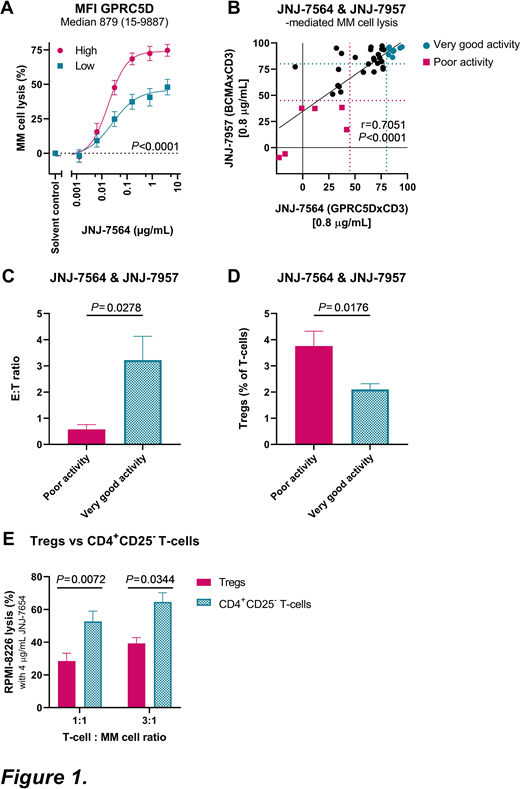
Results: We found no difference in JNJ-7564 efficacy with respect to disease stage (NDMM vs DARA-naive RRMM vs DARA-R MM, P=0.48). Importantly, the presence of high-risk cytogenetic abnormalities [del(17p), t(4;14) and t(14;16)] did not impair JNJ-7564 efficacy. The level of target expression was an important determinant of response, as evidenced by superior MM cell lysis in samples with higher than median GPRC5D expression, when compared to lower GPRC5D expression (Fig. 1A). Inferior MM cell lysis was observed in older patients (>67 years), in samples with low T-cell counts or low effector:target (E:T) ratios, and in those with a high frequency of PD-1+ T-cells, HLA-DR+ activated T-cells, or Tregs. These determinants of response also affected JNJ-7564-mediated T-cell activation and degranulation.
To further analyze the impact of Tregs, we performed additional cell line experiments. Purified Tregs impaired T-cell proliferation, and were significantly less potent to kill MM cells when redirected by JNJ-7564, compared to CD4+CD25- T-cells (Fig. 1B). This was accompanied by reduced secretion of IFN-γ, TNF-α, IL-2 and granzyme B.
To evaluate the impact of BM stromal cells (BMSCs) on JNJ-7564 activity, MM cell lines were co-incubated with PBMCs and patient-derived BMSCs. Direct cell-cell contact hampered MM cell lysis, while indirect contact (transwell) did not affect JNJ-7564 activity. Direct contact also decreased secretion of TNF-α and IL-2, and reduced GPRC5D expression on MM cells, contributing to BMSC-mediated resistance to JNJ-7564.
Finally, we simultaneously evaluated the single agent activity of both JNJ-7564 and JNJ-7957 (0.8 µg/mL, dose whereby a plateau in MM cell lysis was observed with both BsAbs) in 40 BM samples. MM cell lysis induced by both agents was strongly correlated (Fig. 1C). In 6 samples, both agents exhibited poor activity (<45% lysis), whereas in 9 samples very good activity was observed (>80% lysis). Comparison of characteristics between these groups showed that a low E:T ratio (Fig. 1D) and high frequency of Tregs (Fig. 1E) significantly impaired efficacy of both BsAbs, suggesting patient-specific factors can determine response to T-cell redirectors targeting different antigens.
Conclusion: We show that tumor-related factors, such as GPRC5D expression, as well as differences in the composition of the BM microenvironment, including E:T ratio, frequency of PD-1+ or HLA-DR+ T-cells or immune-suppressing Tregs or BMSCs, contribute to the variability in response to JNJ-7564. Our data indicate that strategies aiming at optimizing E:T ratio (e.g. induction therapy) or Treg depletion, may improve response to T-cell redirecting antibodies in MM.
Wong:Jhonson & Jhonson: Current Employment. Zweegman:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Verona:Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Adams:Johnson & Johnson: Ended employment in the past 24 months. Mutis:Janssen Pharmaceuticals: Research Funding; Genmab: Research Funding; Takeda: Research Funding; Onkimmune: Research Funding; Gadeta: Research Funding. van de Donk:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Ferrer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal